“Stage 2 – Process Qualification: In the course of this phase, the process style is evaluated to determine if the process is capable of reproducible business producing.” – FDA
To be a Validation Staff Member, your primary skills are your delicate skills. Complex techniques is usually taught – soft expertise are instinctive and so are what is going to set you apart at the conclusion of the working day.
Process validation involves a series of functions happening above the lifecycle in the products and process.
Comprehend several different approaches to implementing particular anticipations of your lifecycle approach to PV like amount of sampling, acceptance conditions and deciding the amount of batches for PPQ/PV.
This segment tells an auditor (and your colleagues) the finalised list of SOPs that are required. This builds over the draft list you supplied earlier.
This screening technique will already have been published through the direct Validation Engineer so when You begin your vocation, your key job website will be to read and recognize what should be completed, then perform the checks based on the method explained and history the outcomes in the right checksheets.
In its advice on process validation, FDA states that process validation is “the gathering and evaluation of information, with the process style phase by means of commercial production, which establishes scientific proof that a process is able to consistently delivering good quality merchandise.”
This protocol incorporates Guidance on how to make the medication and the sort of equipment that is for use in which makes it.
Nonetheless, the most effective preparing for inspections and audits is to make certain high-quality standards are revered in the documentation manufactured every day.
Recall, you ought to under no circumstances indication anything unless you happen to be sure it’s an correct reflection of the read more situation – and you'd under no circumstances indicator nearly anything that was not your personal operate.
Limit possibility of validation failures by Mastering about adequate preparation in process comprehending and ancillary devices.
IQ/OQ/PQ ensures that any tools you use to manufacture your medical system operates just how it must—each time.
For validation personnel, this incorporates ensuring the contents of Validation Summary Reports are in line with the inspectors’ anticipations.
Operational qualification entails tests all the various capabilities in the tools and creating the operational parameters with the gadget, which can include:
 Jonathan Taylor Thomas Then & Now!
Jonathan Taylor Thomas Then & Now! Judge Reinhold Then & Now!
Judge Reinhold Then & Now! Sam Woods Then & Now!
Sam Woods Then & Now! Mason Reese Then & Now!
Mason Reese Then & Now!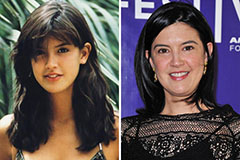 Phoebe Cates Then & Now!
Phoebe Cates Then & Now!